Our Story
The journey from 1999 to Infinity
Our Innovation History
 1999
1999  2002
2002  2005
2005 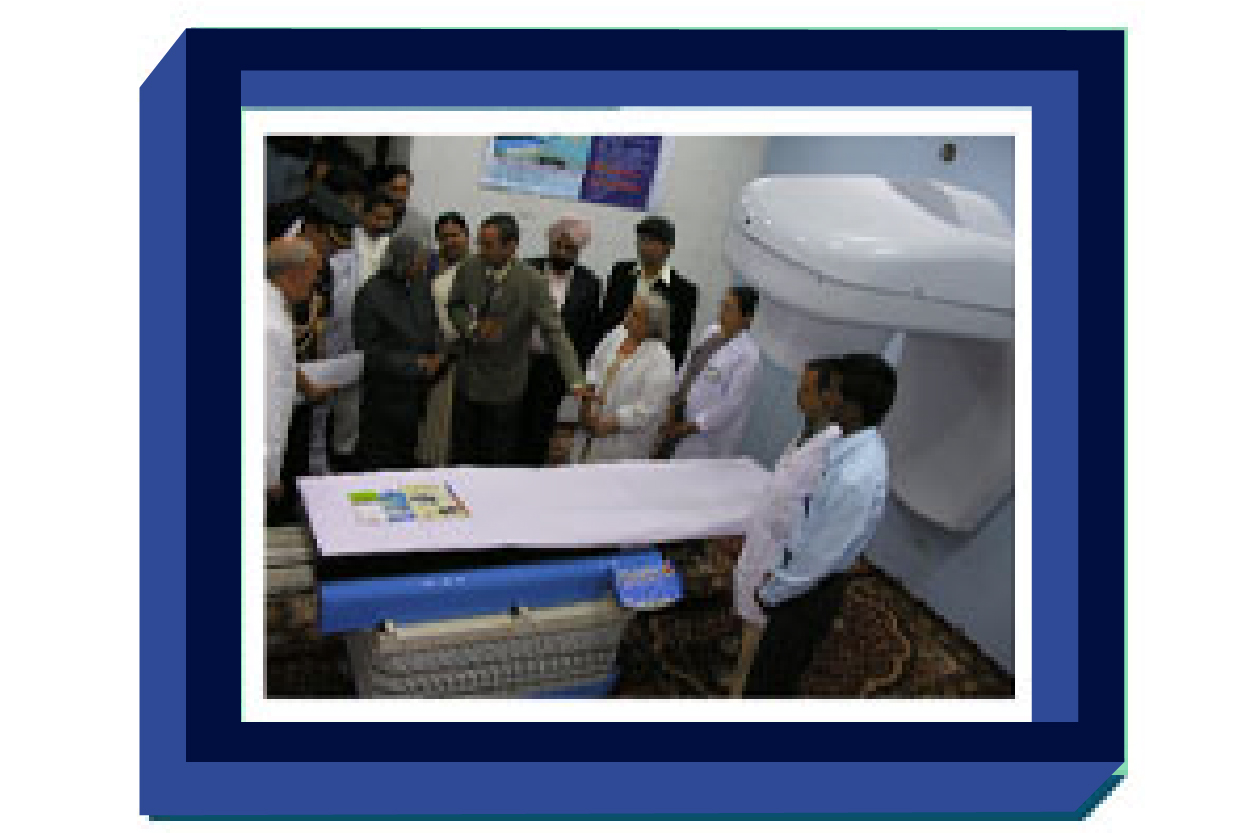 2006
2006  2008
2008  2009
2009  2010
2010  2011
2011  2012
2012  2013
2013  2014
2014  2015
2015  2016
2016  2017
2017  2018
2018  2019
2019  2020
2020  2021
2021  2023
2023 1999
Establishment
In 1999, Panacea Medical technologies was established in Whitefield, Bangalore by a group of Technocrats
2002
Development of MV Linac
In 2002, As linear accelerators gained place in medicine, Panacea began the development of 6MV linear accelerator
2005
Commissioning of Bhabhatron
In 2005, was commissioned at ACTREC, Mumbai
2006
1st Installation
In 2006, Panacea installed its first Bhabhatron II in Indian Red Cross Society Cancer Hospital, Nellore. Andhra Pradesh
2008
Recognition
By 2008, Panacea was ISO certified and gained recognition by INS. Meanwhile, Panacea commercialized its Imagin Simulator
2009
CE Certification
In 2009, Panacea’s Bhabhatron II and Imagin got CE marked. The company was honoured by IERDA award for its contribution to society
2010
Vietnam
In 2010, Panacea installed Bhabhatron II in Vietnam, making it “the first international installation”
2011
R&D Facility
In 2011, DSIR recognized Panacea as an R&D facility
2012
Excellence
In 2012, Panacea was honored with the India SME excellence award in recognition of exemplary achievement in “Manufacturing sector”
2013
Quality Award
In 2013, Panacea Received quality award by Quality Council of India as recognition of Bhabhatron II’s success for cancer care
2014
Treating Lives
With the donation of Bhabhatron II and Imagin to Mongolia, Bhabhatron II had treated more than 100,000 patients by 2014
2015
66th Republic Day
In 2015, A proud moment for Panacea when Bhabhatron II was displayed on Tableu on 66th Republic Day parade
2016
Improving Access
By 2016, Panacea had left no stone unturned to improve access to radiotherapy with Bhabhatron II installed in Kenya, Tanzania and Kyrgyztan
2017
Kassia Award
In 2017, For its contribution, Panacea was awarded with Kassia Ujwala Prashasti Award and was certified with ZED
2018
Lilac – CE
In 2018, Bhabhatron II was installed in Uganda and Madagascar, ensuring timely treatment of cancer in these unreached areas. With Lilac getting CE marked in 2018, Panacea had empowered clinicians in the early detection of breast cancer.
2019
Linac – CE
In 2019, Panacea’s Linear Accelerator was CE marked. Panacea also received patent for its Linac’s In gantry dual kV imaging
2020
Expanding Horizon
In 2020, Panacea setup its a new state-of-art manufacturing plant in Malur and started its US operations in Indiana, USA as part of its expansion plan to cater to the increasing needs of Radiotherapy. Panacea also installed Bhabhatron MLC in Varanasi and Madurai
2021
USFDA 510(k) received for Siddharth-II
In 2021, Panacea received USFDA 510(k) clearance for Linear Accelerator- Siddharth-II.
2023
Type approval & clinical licence received for Siddharth-II
Panacea received Type Approval and Clinical Licence from AERB for its Linear Accelerator- Siddharth-II in 2023. Panacea also received US 510(k) clearance of Siddharth-II integrated with RFA.
BOARD OF DIRECTORS

Mr. G.V. Subrahmanyam
(Co-Founder, Managing Director and Chief Technology Officer)

Mr. Y Ramann
(Director - Finance)

Mrs. Valli
(Executive Director)

Mrs. Bala Deshpande
(Member of Board of Directors)

Mr. Tarun Sharma
(Member of Board of Directors)

Mr. Charles-Antoine Janssen
(Nominee Director)
